A case of encephalitis following COVID-19 vaccine – Journal of Infection and Chemotherapy (jiac-j.com)
Abstract
We describe the first case of encephalitis following coronavirus disease 2019 (COVID-19) vaccination. Our patient was a 46-year-old Japanese woman who presented with acute onset diplopia. Subsequent magnetic resonance imaging revealed brain stem encephalitis that was rapidly responsive to high dosage steroid therapy and completely improved. Although the occurrence of encephalitis after vaccination could have just been a casual temporal association, her symptoms were temporally correlated with two vaccinations. Our case suggests caution and indicates treatment and prognosis, despite no evidence of a causal relationship. Nonetheless, this report emphasizes the enormous benefits of vaccination, which should not be undermined.
1. Introduction
The neurotropism of the coronavirus disease 2019 (COVID-19) has been well known, with reports showing that COVID-19 can cause neurological diseases such as acute transverse myelitis (ATM), neuromyelitis optica (NMO), acute disseminated encephalomyelitis, and acute motor axonal neuropathy [1,2, 3]. However, the mechanism by which it causes these diseases remains unclear. The Centers for Disease Control and Prevention (CDC) Vaccine Adverse Event Reporting System related to COVID-19 vaccines had reported that as of September 9, 2021, 66 cases had developed encephalitis [[4]] However, the details of such cases have not been revealed. We herein present a case that developed encephalitis after COVID-19 vaccination.
2. Case presentation
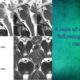
Our patient is a 46-year-old Japanese woman with no previous medical history. After receiving her COVID-19 vaccination [BNT162b2 (Lot EY3860, Comirnaty, BioNTech and Pfizer)], she began having trouble focusing her eyes the next day. Two days later, persist diplopia occurred and gradually worsened, for which she visited a general physician, otolaryngologist, and neurosurgeon. However, given that no significant findings were observed, she was followed up without treatment. Although diplopia fluctuated between improvement and worsening, it persisted without completely disappearing. However, a day after receiving her second dose of vaccination [BNT162b2 (Lot FC5947, Comirnaty, BioNTech and Pfizer)], which was administered 3 weeks after the first dose, her diplopia exacerbated. Five days later, the patient was admitted to our section. On admission, her vital signs and physical examination were normal. Bilateral abduction restrictions were present, whereas other neurological findings were unremarkable. Brain MRI revealed a lesion on the dorsal pons across the midline and no gadolinium enhancement (Fig. 1). Old foci, which could indicate multiple sclerosis, were not detected. MR angiography showed no vascular lesions, and whole-spine MRI was unremarkable. On cerebrospinal fluid examination, total protein content was 53 mg/dL; 3 mononuclear cells/mm3 were detected; glucose was 63 mg/dL, IgG index was 0.5; and oligoclonal bands (OCBs) was absent. Blood tests showed no abnormalities, and serum antibodies to acquaporin-4 (AQP4) and myelin-oligodendrocyte (MOG) tested negative in the cell-based assay. No other abnormalities suggestive of infection, collagen disease, and vasculitis were noted. The patient was thereafter treated with three sessions of high dose methylprednisolone (1 g/day over 3 days, with 4 days off). Subsequently, oral medication was administered starting from 1 mg/kg/day and gradually decreasing. The patient’s symptoms improved, with MRI showing a reduction in the lesions (Fig. 1). No recurrence has been experienced after discontinuing steroid therapy.

 Alternative Health2 years ago
Alternative Health2 years ago
 Life Force Network2 years ago
Life Force Network2 years ago
 Alternative Health1 year ago
Alternative Health1 year ago
 Life Force Network2 years ago
Life Force Network2 years ago
 Alternative Health2 years ago
Alternative Health2 years ago
 Military2 years ago
Military2 years ago